That’s because lithium is in the most electropositive group of elements and sodium/potassium are too reactive for current technology. Theoretically I think Na and K based batteries should perform better as they’re even more electropositive than Li.
(Forgive the spelling error in the picture but it was the simplest one I could find quickly)
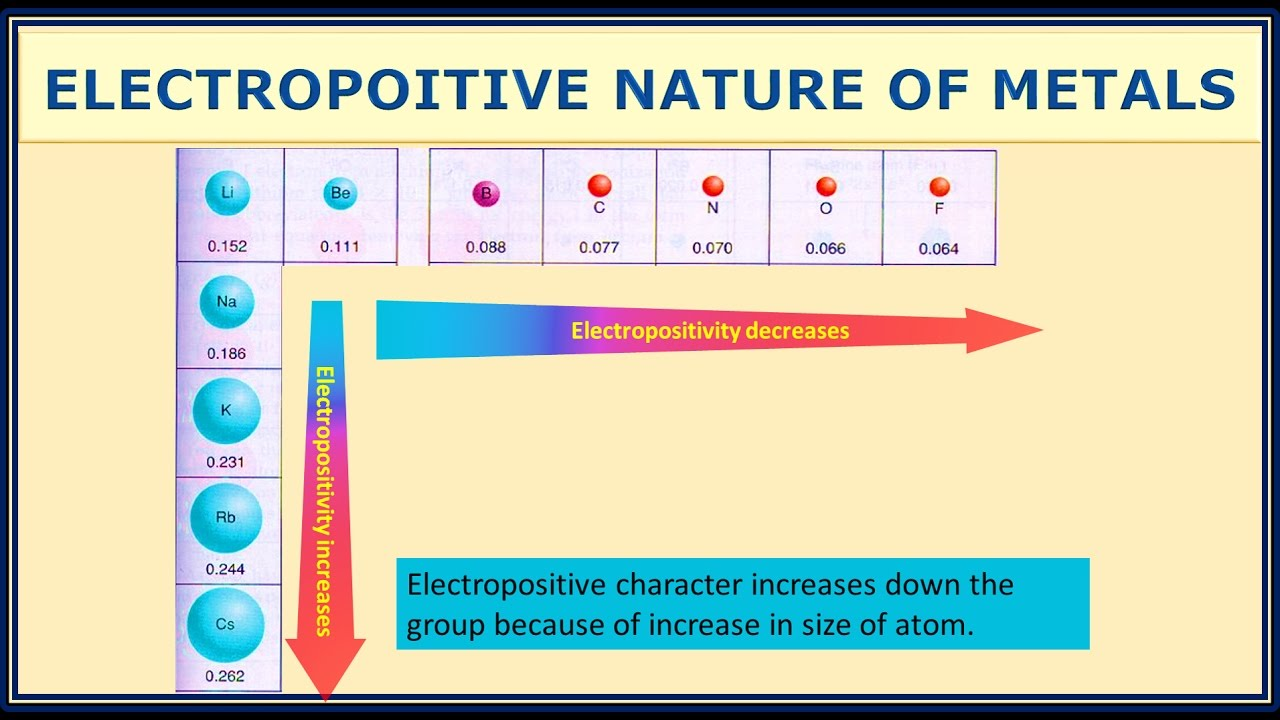
Na and K based batteries should perform better
What I’m hearing is throw some salt on a banana and power my phone for days.
I wasn’t very good at chemistry.
It’s the difference in electronegativity that makes the battery. That’s why you see lithium and oxygen a lot; lithium doesn’t want electrons, oxygen does want them. Sodium and potassium are very close in electronegativity so the salty banana battery wouldn’t be good.
I’m waiting for the cesium / fluorine battery, should theoretically be awesome. Or extremely explosive
That’s a much more serious and informative answer than I deserved.
Thank you for the explanation.
Gotta put my chemistry education to good use somehow, certainly not using it in the IT career I ended up getting in.
-I’m waiting for the cesium / fluorine battery, should theoretically be awesome. Or extremely explosive
I wonder how much it would cost to personally attempt this experiment… (starts hunting for renters insurance)
The other thing for lithium is that its light, VERY light, which of course is ideal for hand sets. Manufacturers love the light and slim designs even though consumers would prefer to have a handset that can go 7 days without a charge



